Here is a quick summary on probiotics and allergy as reported by two meta-analysis. The first one is Cuello-Garcia 2015 in JACI
Probiotics used by pregnant women or breast-feeding mothers and/or given to infants reduced the risk of eczema in infants; however, the certainty in the evidence is low.
the second by Zuccoti 2015 in Allergy
Seventeen studies, reporting data from 4755 children (2381 in the probiotic group and 2374 in the control group), were included in the meta-analysis. Infants treated with probiotics had a significantly lower RR for eczema compared to controls […]. No significant difference in terms of prevention of asthma, wheezing or rhinoconjunctivitis was documented.
Unfortunately the eczema effect fades out with increasing age:
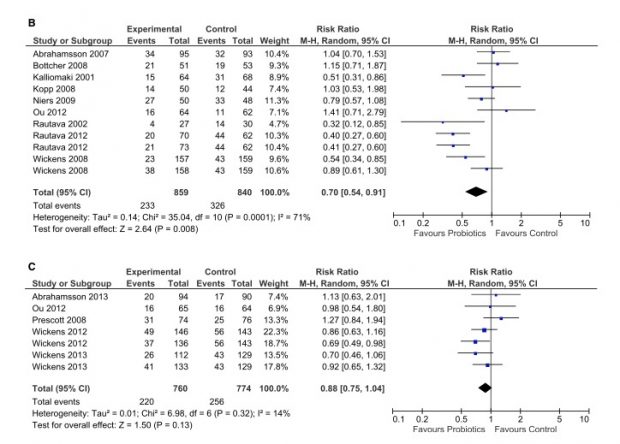
An older Cochrane review reported
The evidence suggests that probiotics are not an effective treatment for eczema, and probiotic treatment carries a small risk of adverse events.
So it is not unexpected what experts say
Probiotics do not have an established role in the prevention or treatment of allergy. No single probiotic supplement or class of supplements has been demonstrated to efficiently influence the course of any allergic manifestation or long-term disease or to be sufficient to do so.
Also the European Food Safety Authority remains sceptical with an interesting argument
One EU regulation that makes it hard to demonstrate the benefits of probiotics is the prohibition of medical claims, i.e. claims that a food prevents or cures a disease. If this prohibition did not exist, manufacturers of nutritional treatments might circumvent the costly procedures required for drugs, and market their products to ill people without thorough proof that they are effective and safe.
Remember, you may die from probiotics, as shown by Besselink 2008
In patients with predicted severe acute pancreatitis, probiotic prophylaxis with this combination of probiotic strains did not reduce the risk of infectious complications and was associated with an increased risk of mortality. Probiotic prophylaxis should therefore not be administered in this category of patients.
or the CDC Health Advisory 2014
Solgar ABC Dophilus® Powder should not be used [Bifidobacterium lactis, Streptococcus thermophilus, Lactobacillus rhamnosus, possibly contamination with Rhizopus oryzae], especially in infants who may be especially susceptible to infection. In considering the use of any dietary supplement, clinicians should consider that the FDA does not regulate these products as drugs.