There is also a review in the BMJ “Can medical product development be better aligned with global needs?”
Typically, market analyses are performed by pharmaceutical companies. These analyses lead to value propositions and business cases for developing new products based on technologies those companies have either developed, or for which they have licensed intellectual property. These analyses—together with assessments of “end-user” (patient) preferences, and assessments of regulatory pathways—drive research and development (R&D) investments. In traditional for-profit product R&D, the unmet medical need is factored in only partially, including through the end-user preferences and the company’s assessment of likely regulatory authority perspectives. In some cases, governments or multilateral agencies can be large scale procurers (i.e., they will purchase the product), and in this situation their preferences may be given more weight.
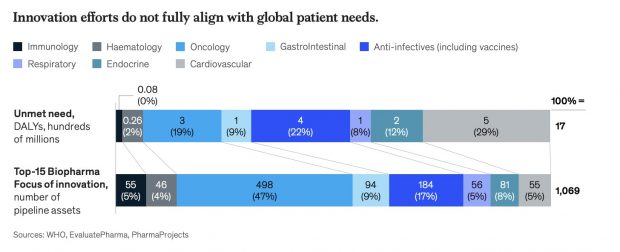
However, at present, only a small proportion of global health R&D spending (around 2%) is on the compelling medical problems faced by LMICs.