Mutation accumulation in the human genome is a largely neglected research field. Most mutations have a very small effect (if any) and may be compensated by environmental improvements. I have already argued in that way in my 2003 Triple T paper and will reiterate it soon in PLOS medicine (just found that James Crow 1997 in PNAS and 2000 in nat gen rev had the same opinion). In principle, the improvement of sanitation and better medical care is leading to a retention of mutations that would be otherwise subject of purifying selection.
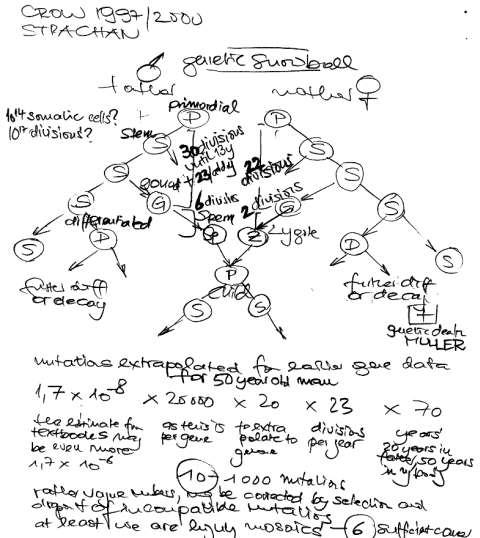
Another important factor seems to be the increase of parental age in Western societies. A 20 year old man had about 150 chromosome replications while a 40 year old had about 610 replications. To count the number of your somatic mutations, you need to add all events of your lifetime plus the age of your father at birth minus 9 months … Even with the high fidelity of polymerases, DNA replication remains an error prone process leading eventually to an increase of germline mutations (as may be seen with achondroplasia, Apert syndrome, neurofibromatosis and prostate cancer). With the increasing age of fathers we are now nearly doubling the absolute number of mutations every generation – and we keep them in the pool in contrast to previous centuries. Crow in PNAS 1997 even said
I do regard mutation accumulation as a problem. It is something like the population bomb, but it has a much longer fuse
Addendum 20 Nov 2013
In another post, I detailed the 3,93 figure derived from cancer tissues. The best human estimate at the moment is in this Cell paper that shows a rate between 2.0 and 3.8 x 10^-8 cells. Sperm sequencing may not represent a good model as there are too many degenerate cells.
Addendum 1 Jan 2021
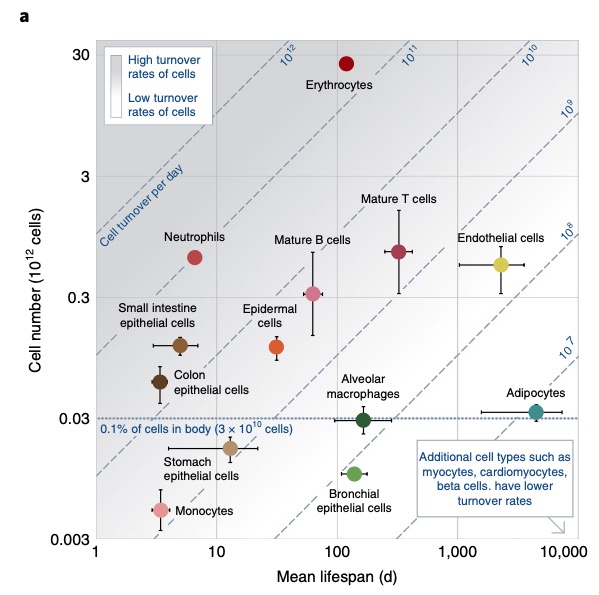
Here is a new nature medicine paper on cell turnover.
What I do not understand – shouldn’t we have a much higher leukemia rate in the population? Leukemia is only on the11th place.
Addendum 29 Apr 2021
Another Nature study shows
Differentiated cells in blood and colon displayed remarkably similar mutation loads and signatures to their corresponding stem cells, despite mature blood cells having undergone considerably more divisions. We then characterized the mutational landscape of post-mitotic neurons and polyclonal smooth muscle, confirming that neurons accumulate somatic mutations at a constant rate throughout life without cell division, with similar rates to mitotically active tissues …
this could be the answer to my previous question
These mutations may result from the interplay between endogenous DNA damage and repair that occurs in cells at all times. The similar mutation burden and signatures in granulocytes and hae- matopoietic stem cells, despite a different divisional load, could also be consistent with a time-dependent rather than a division-dependent accumulation of somatic mutations during haematopoiesis
although it will need independent corroboration before making any conclusion that damage repair is more important than replication.