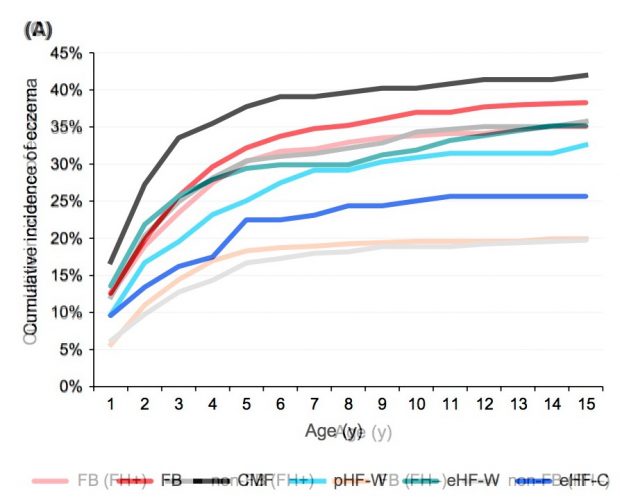
A new analysis of the GINI-LISA study published this month in Pediatr Allergy Immunol has an interesting plot showing the eczema incidence in the first 15 years of life. Eczema is a major allergy symptom.
On the right side (FIG1C), we find the untreated group, and on the left (FIG1A) we find the intervention group. Overlying both figures with Photoshop gives an interesting perspective.
When we use the lower opaque double line as reference, all types of baby feeding give an increased eczema risk. To be more comparable, the opaque upper two lines should be used, which will result in zero effect size.
The question is: Why is breast feeding / breast milk (BF) a risk factor in the interventional (left figure) but not in the observational arm (right figure)?
- In accordance with national and international guidelines full BF was recommended for 4 months in the interventional group. “Only in case of insufficient BF, children were randomized to one of the baby foods”. “Insufficient BF” is not clearly defined and the reasons seem to be complex.
- The general pediatric recommendation at the time of the study was that BF is allergy-preventive and that allergic children should be BF as long as possible.
- BF in the interventional arm therefore is a self-selected biased group primarily by allergic mothers and children with unclear criteria. The authors acknowledge in the discussion “Even adjustment for the potential confounders does not eliminate the phenomenon of reverse causality. Particular early eczema occurring in the first months of life may influence the mother’s decision regarding full breastfeeding versus formula supplementation.”
- BF is a complex chemical mixture that even varies over time – there is is a long-standing controversy on the effect of BF on allergy with many negative, null and positive associations to allergy. From this discussion we can assume, that there is probably no direct effect of BF on allergy development. More likely BF is a proxy for something else – setting up a further biological argument that the interventional BF group should NOT be used as a reference.
Children who receive “antiallergic” formula food will not have any benefit which is in accordance with a recent review in the BMJ. Nevertheless Nestlé Beba Pro HA is further advertising as “reducing allergy risk to milk protein”, Hipp HA Combobiotik as “reducing allergy risk by strong protein cleavage”, Nutramigen 1 LGG as “extensively hydrolysed, hypoallergenic formula … suitable for babies with … allergy”.
Postscriptum
The revised blog entry has been published as a letter to PAI. Unfortunately it turned out only after submission that there are also some major issues with the number of individuals included when comparing the 2003 with the 2013 and 2018 report.