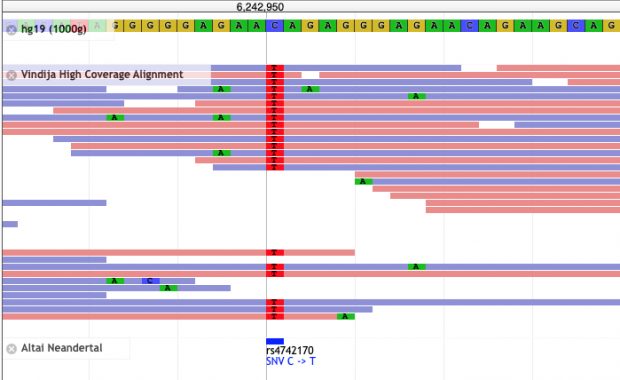
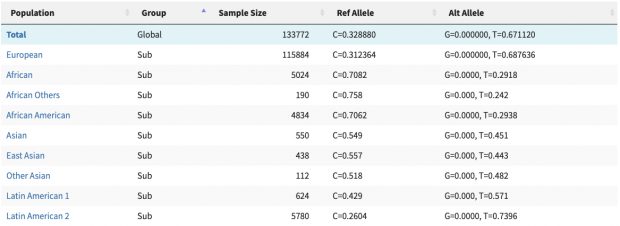
New work by Harvard colleagues shows how sunshine hormone D constrains inflammation by modulating the expression of key genes on chr17q. It builds on earlier collaborative work on the vitamin D receptor in 2004 (see their ref 5) as well on my annotation of IKZF3 (aka aiolos aka god of winds) in 2008 and again in 2022.
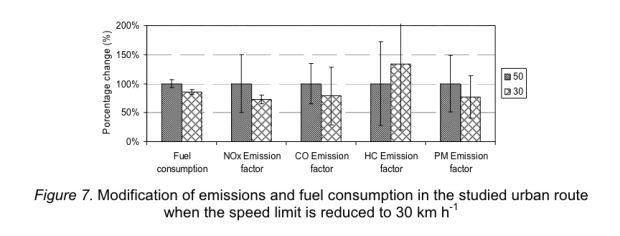
While our focus on allergy development was on vitamin D supplementation of newborns, the interest of Weiss et al. was on vitamin D deficiency in pregnancy. Vitamin D deficiency may not be attributed to the rise of the asthma and allergy epidemic although this remains the never ending obsession of Weiss et al.
Nevertheless, also a wrong hypothesis may lead to new insights. IKZF3 clearly is a key player where more recently heterozygous missense/LOF variants have been found in families with B-lymphopenia and EBV-associated lymphoma while the allergy proning effect is more in the 5-prime region.
The new study shows (again) that cholecalciferol suppresses the activation of the IL-2 pathway. But what is the net effect of artifical cholecalciferol exposure on naive T cells? Unfortunately the new paper narrowly focuses on cytokine production in Th2 cells only and even misses the famous Cantorna review that clearly says
Since 1983 it has been described that 1,25(OH)2D inhibited T cell proliferation and the secretion of select cytokines after mitogen stimulation. Moreover, 1,25(OH)2D directly inhibited IL-2 and IFN-γ transcription [17,18]. More recently 1,25(OH)2D has also been shown to inhibit IL-17 secretion by Th17 cells. The effects of 1,25(OH)2D on Th2 cells is more controversial with evidence that 1,25(OH)2D inhibits IL-4 transcriptionally as well as evidence that 1,25(OH)2D upregulates IL-4 in mouse and human T cells.
So we need to rephrase the finding of an “immune protective effect of vitamin D in allergic lung inflammation” to an overall “immune suppressive effect of vitamin D” which is basic textbook knowledge. Unfortunately the early origin of allergy induction remains a mystery.