This content has restricted access, please type the password below and get access.
Category Archives: Allergy
Second look: Again U-shaped vitamin D effects
It is again an U, no doubt.
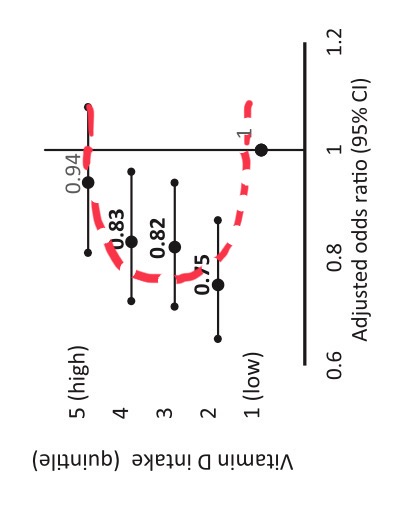
we have now at least 36 studies…
PPPR
PPPR is not only the abbreviation of “pandemic preparedness prevention research” but also of “post publication peer review”. PPPR is particular important in a scenario described in an excellent commentary of an excellent article that highlights the strategy
for a “team of rivals” in which you “invite your academic rivals to work with you.” It still depends too much on the goodwill and honesty of people with too much to lose. Also, Kahneman, who is quoted/paraphrased, seems to be missing the point when he says that “With competing hypotheses and theories in play, the rivals will quickly spot flaws such as hypothesis myopia, asymmetric attention or just so storytelling, and cancel them out with similar slants favouring the other side.”
No, they wont’t, they would have to leave the club. Without independent PPPR any bias cannot be discovered as can be seen in the classical examples of synchronized groups in GWAS or farming studies – crushing opponents by hugging.
Farming and allergy
Hopefully the thread here on “farming and allergy” is now coming to an end. I tried to refute it for many years – last attempt in 2020
the main objection against the farming hypothesis is the interpretation of a negative statistical association as a “protective” effect. Only after thorough exclusion of alternative explanations, this interpretation may be justified.
but unfortunately missed another paper from the literature that had already been published in 2011, sorry for that.
With environmental exposures aside, at least 2 profound differences between farming and nonfarming families could be a threat to the validity of such investigations. One of these is the long-term selection of traits and genes in favor of the demanding living conditions of farmers because the agricultural lifestyle is often handed down within families. Referring to the “healthy worker effect,” we could expect certain disadvantages to be underrepresented in a farming population, giving rise to the concept of a “healthy farmer effect.”
Also the second point of Grabenhenrich has never been assessed
The other area expected to be different when comparing farming and nonfarming families constitutes behavioral patterns, lifestyle, and knowledge. For example, “soft factors,” such as health care use, symptom perception, and labeling, as well as access to and interest in health-related information, are most likely to be distributed dissimilarly. This disparity, in turn, could severely affect the response pattern in studies basing their case definition mainly on questionnaires. A temporal shift of these soft factors toward increased cautiousness and awareness has been assumed to contribute to the worldwide increase in symptoms of allergic diseases and, to a lesser extent, to the increase in clinically apparent disease.10, 11 Why should such a change be uniform in all parts of a population? Particular subgroups might be susceptible to catch up more rapidly. This phenomenon could be studied by identifying outliers in otherwise homogenous populations.
in the hope that journalists and politicians will never find it?
Is this “pioneering epidemiology” as judged by MP Dr. Söder?
Promoting a stolen idea (the original was published in German only) where the source was never cited?
Is it any good science ignoring all objections?
True heroes in epidemiology and public health are Semmelweis, Snow, von Pettenkofer, Doll & Hill, Hesse & Rehn but not a MD who can not even explain an Odds Ratio…
An update on the asthma exome
Here is a quick update on some genes of my recent asthma exome paper coming now from the 1 M exome paper published yesterday as a preprint.
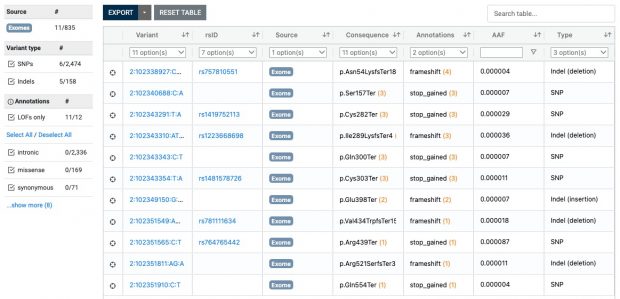
Also ClinVar shows that the IL33 receptor is not “essential” making anti IL33 receptor antibodies like etokimab, itepekimab, tozorakimab a safe therapy although not being effective in any LOF mutation carrier.
The most interesting thing in the preprint is in supplemental table 2 with the s-het values for 16,704 genes. From that table I have selected my favorite target IL33 receptor together with TLR1, ALOX15, GSDMA, IL13 and IKZF3 ( BTNL2 could not be found in the list).
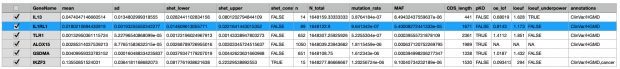
IKZF3 would be dangerous to be touched (see my 2008 commentary) while in the 2022 exome paper I also found only protective variants in the 5′-UTR but not any LOF variant – probably as IKZF3 is the only essential gene in the list.
So what’s next? I am still thinking how to reduce my exome set to the causal variants as half of the mutations are probably LD artefacts. And well, it would be super interesting to examine now two extreme inbred populations for their mutation spectrum, loosing either asthma variants by healthy (Amish) or diseased founders (Tristan da Cunha). Unfortunately there is little hope that this will happen – current science is built more on competition than collaboration.
Y
1905 entdeckte Nettie Stevens das Y Chromosom und die geschlechtsgebundene Vererbung. Wenn das 2023 in Frage gestellt wird, kann man nur auf das lange TAZ Interview aus dem letzten Jahr mit Alexander Korte weiterleiten
Die neurobiologische Forschung ist definitiv den Beleg schuldig, dass Geschlechtsidentität genetisch bedingt sein könnte. Auch aus der Sicht der Entwicklungspsychologie ist es abwegig, davon auszugehen, dass Identität etwas ist, mit dem man zur Welt kommt. Aus meiner Sicht ist Identität stets das Resultat einer individuellen Bindungs- und Beziehungs- und auch Körpergeschichte.
Any proof for hygiene hypothesis by lockdown babies?
It is an interesting question – does social distancing influence later allergy ? Lawler et al. in November 2021

report the impact of COVID‐19 lockdown in 365 Irish babies at 6 months of age enrolled in the CORAL study. These were a subset of 3773 infants born in two participating major maternity hospitals in Dublin between March and May 2020. Unfortunately only 10% of children participated, so families were self-selected. Allergic rhinitis was common in both mothers (36%) and fathers (30%) which is higher than reported by Allergy Ireland (26%) and explained by the authors that parental allergy is “higher than the general population, which may have contributed to parental desire to enrol in this study.”
A follow-up in March 2022 in 344 children consecutively shows higher food allergy (4.7% vs 3.5%, NS) when compared to an earlier cohort (BASELINE 2008). Atopic dermatitis increased of 15.5% in the BASELINE study to 25.3% in CORAL (no P reported) which is not unexpected given the interest of parents in an allergy study.
Maybe a short questionnaire at 2026 school entry would be informative than the current study? Nevertheless the authors needed now to analyze their stool samples sitting in the shelves. This is the content of the March 2023 preprint that has just been published. There may be an association of some bacteria with atopic dermatitis but in the end it is a useless result as the strongest risk factors for atopic dermatitis – parental history and/or FLG mutations – are missing from the presented models
At the end we can safely assume that the 2020 lockdown did not have any influence on allergy prevalence in Ireland.
Top 5 talks of the 3rd International Summit on Human Genome Editing
The final statement came by email this morning statement-from-the-organising-committee-of-the-third-international-summit-on-human-genome-editing
Remarkable progress has been made in somatic human genome editing, demonstrating it can cure once incurable diseases. To realise its full therapeutic potential, research is needed to expand the range of diseases it can treat, and to better understand risks and unintended effects. The extremely high costs of current somatic gene therapies are unsustainable. A global commitment to affordable, equitable access to these treatments is urgently needed. Heritable human genome editing remains unacceptable at this time.
And well, my subjective selection of the best talks is also here (unfortunately the video quality is poor and there is no way to timestamp the URL, so you have to recall the time marks below).
As always masterful moderation by Robin Lovell-Badge including the fire alarm ;-) My top 5 talks are
- Chinese legislation whitewashed at 1:03:10
- David Liu excellent overview at 1:25:14
- Filippa Lentzos with a super nice talk on hopes and fears at 2:53:25
- Tue Kiran Musunuru with another excellent overview at 2:18:54
- Rising star Tina Rulli at 1:28:29
(Jennifer Doudna 4:25:17 was a bit disappointing when talking about my research field involving asthma and microbiome.
The two father mouse of Katsuhiko Hayashi that gained wider attention [Guardian, Nature, …] is found at 3:14:02.
Prodomo
Authorities should protect infants and parents from commercial interests
An editorial linked to a new BMJ study on baby food writes
Cheung and colleagues reports a survey conducted in 15 countries exploring the health and nutrition claims made in the marketing of infant formula products. The authors performed a systematic search of websites, examined packaging of formula products, and documented claims made about the formula product and citations of scientific evidence supporting those claims…
Health professionals and families lack the time to properly scrutinise claims. Industry is unlikely to change its practice given shareholder interests. Self-regulation has not worked, and responsible, ethical marketing by the formula industry seems unlikely.
As mentioned here numerous times – irresponsible marketing, distorted studies, corrupt pediatric scientific societies – it is a never ending story.
Warum es in der DDR so viel weniger Allergien gab
Ich hatte direkt nach der Wende in Halle und Leipzig die erste Ost-West Allergiestudie* durchgeführt und dabei die verblüffende Entdeckung gemacht, dass es im Osten nicht mal halb so häufig Allergien gab.

Der Grund dafür war uns zunächst nicht klar, aber dann stelle sich doch bald heraus, daß es wohl die unterschiedliche Vitamin D Prophylaxe war – im Westen gab es tägliche niedrige Dosen ab der ersten Lebenswoche, im Osten wurde ab dem 2. Monat wenig hohe hohe Einzeldosen verabreicht. Wenn Kinder im Osten krank waren, dann fiel auch immer wieder die eine oder andere Dosis aus, auch wurde das Schema nach meiner Recherche nicht immer komplett durchgezogen. Leider haben wir uns damals aber nur die Impfpässe angesehen, nicht aber die im Nachinein wichtigeren Wiegekarten.
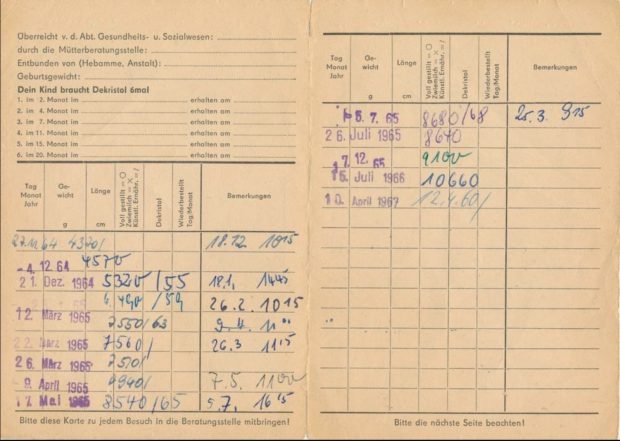

Die Befürchtung der Professoren Mai und Beuren in dem alten SPIEGEL Artikel über Vitamin D Nebenwirkungen haben sich zum Glück nicht bewahrheitet. Dafür aber stellte sich dann aber eine unerwünschte immunologische Wirkung heraus die damals noch nicht bekannt war.
Mit dem aus England bzw Amerika importierten Schema zur Supplementierung stiegen die Allergien an. In der BRD war das ab den frühen 60er Jahren , wie überhaupt die englischsprachigen Länder immer die höchsten Allergiehäufigkeiten hatten, da sie wegen der häufigen Rachitis auch viel konsequenter supplementierten (die Rachitis hiess früher einmal auch “englische Krankheit”).
Mit dem Mauerfall 1989 setzte der Anstieg dann auch in der ex DDR ein und erreichte nach 10, 20 Jahre das Westniveau . Leider wurden unsere Warnungen vor einer zu frühen Supplementierung nicht ernst genommen, der Osten Deutschlands hat – wie so vieles andere auch – das Schema aus dem Westen übernommen und den Preis dafür mit ebenfalls hohen Allergieraten bezahlt.
Der Mechanismus der Allergieentstehung ist dabei nur teilweise aufgeklärt: Vitamin D ist jedenfalls immunsuppressiv mit vielfacher Auswirkung auf B und T Zellen was seit dem Nachweis des Vitamin D Rezeptors auf diesen Immunzellen wissen. Die Supplementierung stört offensichtlich die initiale Klassifikation ob ein Protein harmlos oder allergen ist.
Warum es aber auch schon 1989 Allergien im Osten gab? Nun, es war ja keine Vitamin D freie Zone, offensichtlich sensibilisiert man sich auch noch ausserhalb des überkritischen Intervals in den ersten Lebenswochen.
Allergy GWAS hits in VDR binding sites
It seems that I missed an interesting 2017 paper that looked for disease-associated SNPs in canonical DR3 motifs. Only 7 out of 211 traits showed significant hits, one of these was self-reported allergy. When annotating these SNPs, there are only two genes: LINC00299 and TLR1
hg38 position
rs10174949 2:8302018 LINC00299
rs10178845 2:8303773 LINC00299
rs5743566 4:38804221 TLR1
rs2101521 4:38809830 TLR1
rs5743565 4:38804262 TLR1
rs45588337 4:38805607 TLR1
rs55830619 4:38805643 TLR1
So are TLR1 & LINC00299 variant carriers more susceptible to vitamin D induced allergy?
LINC00299 (Long Intergenic Non-Protein Coding RNA 299) is a RNA Gene of largely unknown function, associated so far with allergy only on a genetic level in Framingham, href=”https://pubmed.ncbi.nlm.nih.gov/23817569/”>23andme and other studies. We don’t know so much here, the function of the long non coding RNAs
depends on subcellular localization. Depending on their niche, they specifically interact with DNA, RNA, and proteins and modify chromatin function, regulate transcription at various stages, forms nuclear condensation bodies and nucleolar organization. lncRNAs may also change the stability and translation of cytoplasmic mRNAs and hamper signaling pathways. Thus, lncRNAs affect the physio-pathological states and lead to the development of various disorders, immune responses, and cancer.
The TLR1 genetic association is found by many genetic studies, while the clinical association is probably more by an infectious origin. TLR1 is a pattern recognition receptor with a specificity for gram-positive bacteria and also included in my forthcoming exome paper as a protective factor for asthma/allergy. And we are also close to my earlier review of vitamin D, the microbiome and allergy…
Does any co-infection response during first vitamin D exposure influence allergic sensitisation? There are indeed some hints of an short-lived effect of lung group 2 innate lymphoid cells (ILC2s)
Laboratory mice cohoused for 2 weeks had impaired ILC2 responses and reduced lung eosinophilia to intranasal allergens, whereas these responses were restored in mice cohoused for ≥2 months. … These findings suggest that ILC2s respond dynamically to environmental cues and that microbial exposures do not control long-term desensitization of innate type 2 responses to allergens.
Rotten Baby Milk
I have criticized in the past the milk industry sponsored research that is now operating at a similar scale like research funded by cigarette industry [1]. And well, it seems that I am not alone here when reading a new CEA paper “Are paediatric allergy services promoting or harming public health?”
There were increasing and excessive sales of specialized formula designed for infants with milk allergy—either extensively hydrolysed or amino acid formula. These represented 7.6% of formula sold in 2019 … Although pediatric allergy services cannot be held solely to blame for whole population trends, there is evidence that the clinical guidance used in pediatric allergy clinics might promote or exacerbate these trends. Moreover, many allergy, gastroenterology and pediatric societies and professionals routinely flaunt public health guidance from the World Health Organization by continuing to accept funding from formula companies, despite World Health Assembly resolutions that such conflictive relationships should be avoided.
An absolutely wilde story
In case anyone is interested, there is an absolutely wild story of alleged cheating at the very top of the chess world right now. /1 https://t.co/lStvGHIh8k
— Nïck Brown🌻 (@sTeamTraen) September 7, 2022
Regarding computer help there is an interesting video
So many asthma papers under fire
As an avid PubPeer reader, I found a new entry by Elisabeth Bik recently about Andreas Pahl of Heidelberg Pharma who has already one retracted and several more papers under scrutiny.
Unfortunately there are now also many asthma trash papers from paper mills. Another example was identified by @gcabanac, distributed by @deevybee and published at Pubpeer.
In total there are 386 asthma entries at PubPeer. What is really happening in this field? When I started the field there was just one misconduct case – Ranjit Kumar Chandra. That’s an increase from 1 to 386…
