(first published 12 Dec2020 and revised 10Dec2021)
We had a major discussion right before our 2010 paper where I argued that rare variants should have been included into our asthma/allergy/dermatitis GWAS. Ten years after there is now a nice paper using massive exome sequencing that finally includes them.
It seems that the respiratory tract isn’t so much influenced by rare gene variants but that there is a strong effect in the immune system.
And there is another interesting fact.
…Surveying the contribution of rare variants to the genetic architecture of human disease through exome sequencing of 177,882 UK Biobank participants …if we look at the …. European population who are carriers of a filaggrin (FLG) PTV, we find those carriers have significantly higher risk for well-known associations, such as dermatitis … and asthma … Concomitant increases in vitamin D levels suggest … increased sensitivity to ultraviolet B radiation.
So far, I have only assumed an asthma/allergy priming effect of oral vitamin D in the newborn gut. This paper now argues for an increased vitamin D sensitivity also in the skin of FLG dermatitis patients which is interesting given the largely contradictory data of serum vitamin D and atopic dermatitis. Maybe dermatologists should focus their research more on skin and local vitamin D turnover?
-II-
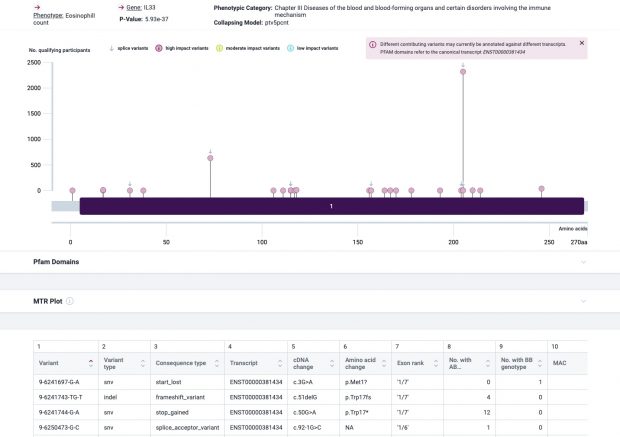
The most prominent IL33 variant carried by over 2,300 people is splice acceptor 9-6250473-G-C followed by 600+ individuals with splice donor 9-6250600-G-T.
There are not too many carriers of this variant by the sheer amount of 177,882 participants. We nevertheless know already something about the seven IL33 splice variants since 2012.
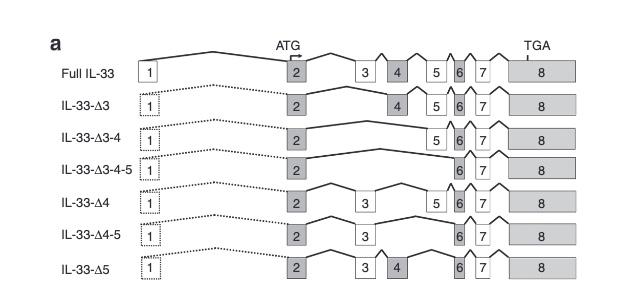
with updates in 2016
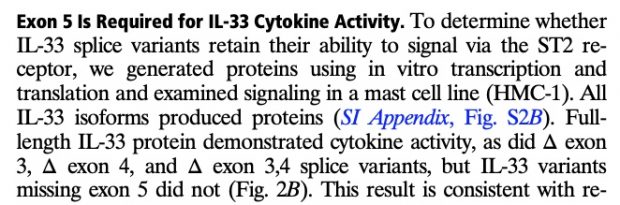
as well as in 2017
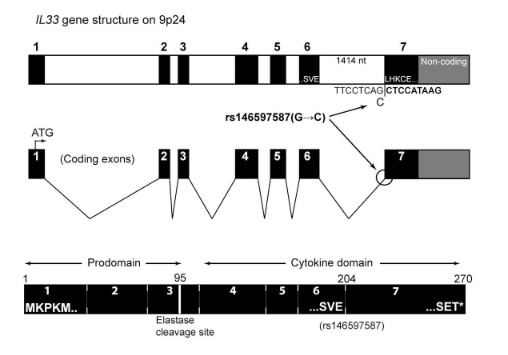
So I did a sequence match to compare the new finding with these older publications.
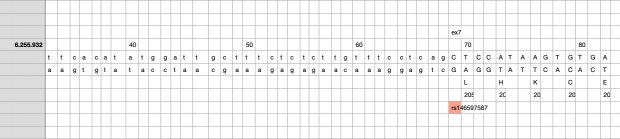
Indeed, the 2017 paper already described rs146597587 which is probably identical to the splice acceptor 9-6250473-G-C in Astra UK Phewas (genome positions do not match – I used hg19 while I don’t know the Astra reference) . Astra says also c.613-1G>C while rs146597587 is just upfront of my codon 205 (3*205=615) whatever that means.
The Astra UK Phewas at least confirms the Iceland paper above
rs146597587-C associates with lower eosinophil counts (ß= -0.21 SD, P = 2.5×10-16, N = 103,104), and reduced risk of asthma in Europeans (OR = 0.47; 95%CI: 0.32, 0.70, P = 1.8×10-4, N cases = 6,465, N controls = 302,977). Heterozygotes have about 40% lower total IL33 mRNA expression than non-carriers and allele-specific analysis based on RNA sequencing and phased genotypes shows that only 20% of the total expression is from the mutated chromosome. In half of those transcripts the mutation causes retention of the last intron, predicted to result in a premature stop codon that leads to truncation of 66 amino acids.
So it is basically a rediscovery meaning that we reached saturation.