The Tristan da Cunha asthma study was leading to patent US6087485A. The patent describes
highly significant linkage in the genome scan (p=0.0001 for history of asthma and p=0.0009 for methacholine challenge) … at D11S907, a marker on the short arm of chromosome 11.
D11S907 or AFM109YA1 is a microsatellite marker located at 11p13 in a gene known as EHF (ETS homologous factor). There should be 2 genes in close proximity of the marker: ASTH1I and ASTH1J.
ASTH1I and ASTH1J were detected by exon trapping. ASTH1I exons detected a 2.8 kb mRNA expressed at high levels in trachea and prostate, and at lower levels in lung and kidney …
ASTH1J exons detected a 6.0 kb mRNA expressed at high levels in the trachea, prostate and pancreas and at lower levels in colon, small intestine, lung and stomach.
The sequences of table 2 in the patent are sufficient now to locate ASTH1I and ASTH1J.
Using these primer sequences, I find a perfect sequence match in ELF5 (=ASTH1I) and EHF (=ASTH1J).
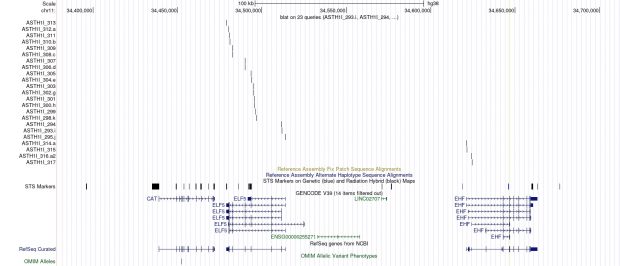
EHF leads to a 42 kb and ELF5 to a 35 kb transcript. So both genes are clearly identified but there is not any link to asthma genetics that has been verified at a later stage.
Why were all associations that have been published later from this study not in this region but on 13q (CYSLTR2) or 1q (DENND1B)? Is there something wrong with the linkage scan?
We don’t know but there could be too many inbred loops that were not adjusted for in the linkage analysis.
The first people to settle and raise families were three soldiers who stayed on the island when the British garrison was withdrawn in 1817. The best known of these men was a Scot William Glass, who is regarded as the founder of the present Tristan community. He married a woman from Cape Town and they had 16 children … The level of inbreeding is so high that all the islanders are cousins and on average two siblings are cousins according to 50 different pathways.
Even if we believe that the linkage was correctly calculated, the sample size was limited and p values clearly not convincing. 274 polymorphic microsatellite marker were more at the lower end of a marker panel at that time. Statistical analyses was conducted using Haseman and Elston algorithm assuming a dominant mode of transmission with incomplete penetrance which may also not be adequate for a complex disease.
The Toronto cohort included 59 small families having at least one affected individual. These were ascertained based on the following criteria: (i) an affected proband; (ii) availability of at least one sibling of the proband, either affected or unaffected; (iii) at least one living parent from whom DNA could be obtained. A set of 156 “triad” families consisting of an affected proband and his or her parents were also collected.
A multiallelic check of the sequenced variant could have shown already at that time that another gene in linkage disequilibrium was responsible for the effect (results of neighboring marker are not reported in the patent). Today we know of only one candidate gene on chromosome 11 (at EMSY / LINC02757) which is about 40 MB far away. While 1MB linkage disequilibrium is possible in an inbred population effects over 40 MB are somewhat unlikely.
So there is no other asthma gene around that would explains the linkage found. Is ELF5 really a false finding? Digging a bit deeper into ELF5 ( also known as ESE2), it seems that it is an epithelium derived transcription factor regulating the differentiation of terminal lung as described in 2008.
We found that expressing high levels of ELF5 during early lung development disrupted branching morphogenesis and produced a dilated epithelium.
There is also some association with COPD
In the present study, ELF5 showed correlation with differentiation markers, MUC5AC and FOXJ1, indicating that ELF5 may contribute bronchial epithelial differentiation.
and it is a confirmed COVID19 risk factor that is already confirmed
We show that ELF5 is specifically expressed in epithelial cells of the respiratory system, such as secretory and alveolar type 2 cells, using single-cell RNA sequencing and immunohistochemistry.
So the Tristan da Cunha mystery goes on.