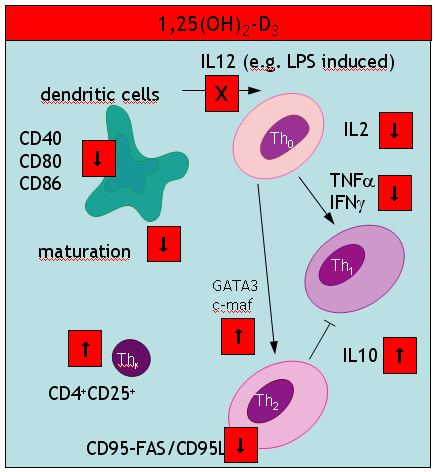
A key event in the vitamin D hypothesis of allergy induction is the immature state of dendritic cells. So far, maturity has been mainly described in terms of reduced expression of cell surface marker like CD80. A new study in Nature now further unravels how the capacity of DC to present antigen may be disturbed. Ubiquitination – the covalent attachment of ubiquitin polymers – of the MHC II ß chain ceases on maturation allowing the transport from endosomal compartments to the plasma membrane. Immature cells seem to be capable to some level of peptide-MHC interaction (at least for some selected antigens) although this process is greatly enhanced by maturation of DCs. Semi-maturity is believed to be an important inetrim stage where at least an earlier review argued
we propose a model in which steady-state migration and partial maturation (semi-maturation) of DCs is embedded as a major component within immune homeostasis, established for permanent and active tolerance induction against self-antigens derived from peripheral tissues by inducing antigen-specific CD4+ Tr cells. Semi-maturation induced by proinflammatory cytokines, such as TNF-alpha, seems to represent a unique developmental tolerogenic stage for DCs, which is based on the absence of proinflammatory cytokine production, despite high expression of MHC II and costimulatory molecules.
Another interesting study in the J Immunol – coined “alternatively activated dendritic cells” the authors probably talk about the same immature cells (compare with my cartoon summarizing a 2002 paper in Trend Mol Med). These immature DCs secrete high levels of IL10 (a paradox discussed in my most recent paper). In addition these DCs produce low amounts of IL12p70, TLR4 and CCR7. What was new to me, was an impressive list of pharmacological agents that suppress DC development: aspirin (also paracetamol?), corticosteroids, cyclosporine A, rapamycin (also other antibiotics?), and finally mycophenolate mofetil.
There is also an update of the IL10 paradox: Allergic sensitization may be down regulated by CD40 AGONISTs independent of IL10!
Finally, I would like to understand what immature really do after encountering allergen exposure. A new paper in nature immunology says that
immature DC are also thought to carry antigen to lymph nodes and to interact with naive T cells but without a previous maturation stimulus, those interactions result in abortive activation of the T cells, which can be eliminated, rendered unresponsive or induced to differentiate into regulatory T cells.
which still does not answer my question.
Vitamin D actions
Yea, yea.